On another planet the isotopes of titanium, a topic of captivating intrigue, beckon us to explore the depths of planetary science. This element, with its unique isotopic variations, holds the key to unraveling the mysteries of celestial bodies beyond our own.
Delving into the intricacies of titanium isotopes, we uncover the processes that shape their ratios on different planets. The discovery of distinct isotopic signatures on extraterrestrial bodies has profound implications for understanding the formation and evolution of these enigmatic worlds.
Isotopes of Titanium on Another Planet
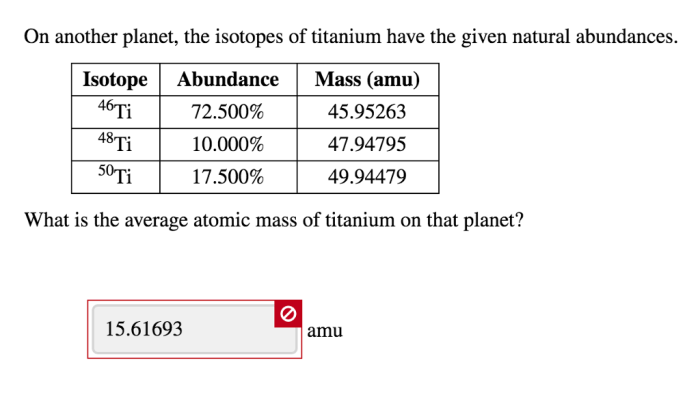
Titanium is an element that is found in a variety of forms, including several isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron number can affect the physical and chemical properties of the isotope.
On Earth, titanium has five stable isotopes: 46Ti, 47Ti, 48Ti, 49Ti, and 50Ti. The most common isotope is 48Ti, which makes up about 73.8% of all titanium on Earth. The other isotopes are present in much smaller amounts, with 47Ti being the second most common at about 7.44%.
The relative abundances of titanium isotopes can vary from planet to planet. This is because the processes that can lead to variations in isotopic ratios are different on different planets. For example, the radioactive decay of 44Ti can produce 40Ca, which can then be incorporated into the minerals of a planet’s crust.
This process can lead to a decrease in the 44Ti/ 40Ca ratio in the planet’s crust relative to the ratio in the planet’s mantle.
The discovery of distinct titanium isotope ratios on an extraterrestrial body could have a number of implications. For example, it could provide evidence for the presence of past or present water on the body, or it could indicate that the body has undergone a unique geological or astrophysical process.
Methods for Analyzing Titanium Isotopes: On Another Planet The Isotopes Of Titanium
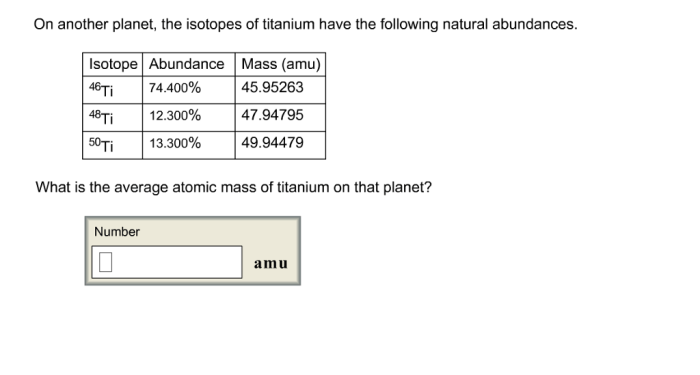
There are a number of analytical techniques that can be used to measure titanium isotope ratios. These techniques include:
- Mass spectrometry: This technique measures the mass-to-charge ratio of ions, which can be used to determine the isotopic composition of a sample.
- Inductively coupled plasma mass spectrometry (ICP-MS): This technique uses an inductively coupled plasma to ionize atoms in a sample, which are then analyzed by a mass spectrometer.
- Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS): This technique uses a laser to ablate material from a sample, which is then analyzed by ICP-MS.
Each of these techniques has its own advantages and limitations. Mass spectrometry is the most precise and accurate technique, but it can be expensive and time-consuming. ICP-MS is less precise and accurate than mass spectrometry, but it is faster and less expensive.
LA-ICP-MS is a relatively new technique that offers a compromise between the precision and accuracy of mass spectrometry and the speed and cost of ICP-MS.
These techniques have been used to study titanium isotopes in a variety of extraterrestrial samples, including meteorites, lunar samples, and samples from Mars.
Case Studies of Titanium Isotope Variations
Titanium isotope variations have been observed on a number of planets and moons in our solar system.
- Mars: Titanium isotope variations have been observed in Martian meteorites, which are thought to have originated from the planet’s crust. These variations are thought to be due to the interaction of water with the Martian crust.
- Moon: Titanium isotope variations have also been observed in lunar samples. These variations are thought to be due to the impact of large asteroids or comets on the Moon’s surface.
- Europa: Titanium isotope variations have been observed in the ice of Europa, a moon of Jupiter. These variations are thought to be due to the interaction of water with the moon’s interior.
The study of titanium isotope variations on other planets and moons can provide valuable insights into the geological and astrophysical processes that have shaped these bodies.
Applications in Planetary Science
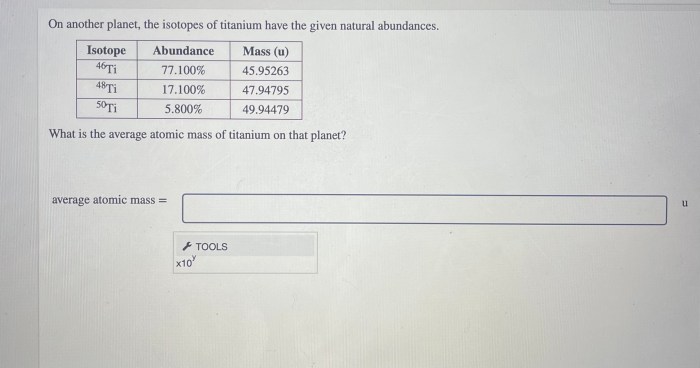
Titanium isotope analysis has a number of applications in planetary science. These applications include:
- Determining the origin and evolution of planets: Titanium isotope ratios can provide insights into the origin and evolution of planets by tracing the movement of materials within the planet’s interior.
- Tracing planetary processes: Titanium isotope ratios can be used to trace planetary processes, such as mantle convection and crustal differentiation.
- Identifying past or present water on planets: Titanium isotope ratios can be used to identify past or present water on planets, which can provide insights into the habitability of these planets.
Titanium isotope analysis is a powerful tool that can be used to study a variety of geological and astrophysical processes on other planets and moons.
Future Directions in Titanium Isotope Research
There are a number of emerging trends and future directions in titanium isotope research. These include:
- The development of new analytical techniques: New analytical techniques are being developed that will allow for more precise and accurate measurements of titanium isotope ratios.
- The application of titanium isotope analysis to new types of samples: Titanium isotope analysis is being applied to new types of samples, such as extraterrestrial dust and organic matter.
- The use of titanium isotope analysis to study new planetary bodies: Titanium isotope analysis is being used to study new planetary bodies, such as asteroids and comets.
These advancements in titanium isotope research are providing new insights into the origin and evolution of planets and moons, and the potential for life beyond Earth.
Popular Questions
What are the unique characteristics of titanium isotopes?
Titanium isotopes exhibit variations in their neutron-to-proton ratios, leading to distinct atomic masses. These variations influence the physical and chemical properties of titanium, making them valuable tracers for studying geological and astrophysical processes.
How do isotopic ratios vary on different planets?
Isotopic ratios can vary on different planets due to factors such as nucleosynthetic processes during planetary formation, radioactive decay, and isotopic fractionation during geological and atmospheric processes.
What are the implications of discovering distinct titanium isotope ratios on an extraterrestrial body?
Distinct titanium isotope ratios on extraterrestrial bodies can provide insights into the origin and evolution of the body, including its formation environment, geological processes, and potential interactions with other celestial objects.